SEMA4D is expressed by many tumor cells as well as by other cells in the tumor microenvironment. In fact, evidence suggests that SEMA4D expression is correlated with tumor aggression and poor prognosis in cancer patients and appears to block immune cell infiltration and activity in tumors.
Preclinical and clinical testing of pepinemab, a monoclonal antibody inhibitor of SEMA4D, has demonstrated immune cell-dependent, anti-tumor activity, characterized by an increase in CD8+ and decrease in inhibitory FoxP3+ T-cells and myeloid suppressor cells (tumor associated macrophage, myeloid derived suppressor cells) post-treatment. Vaccinex has acted on this preclinical validation of pepinemab’s potential for treatment in cancer and has completed a phase 1b/2 trial in combination with Merck KGaA’s anti-PD-L1 checkpoint inhibitor, BAVENCIO® (avelumab). Vaccinex recently initiated a clinical study, in collaboration with Merck (known as MSD outside the US and Canada) to evaluate pepinemab in combination with KEYTRUDA® (pembrolizumab) in recurrent or metastatic head and neck squamous cell carcinoma. We believe there is a strong rationale for treatment of HNSCC because these tumors are known to express high levels of SEMA4D and preclinical studies by Vaccinex and others have indicated that SEMA4D induces increased numbers and activity of myeloid suppressor cells that inhibit immune responses. (Link to clinicaltrials.gov page for this study)
Click to enlarge
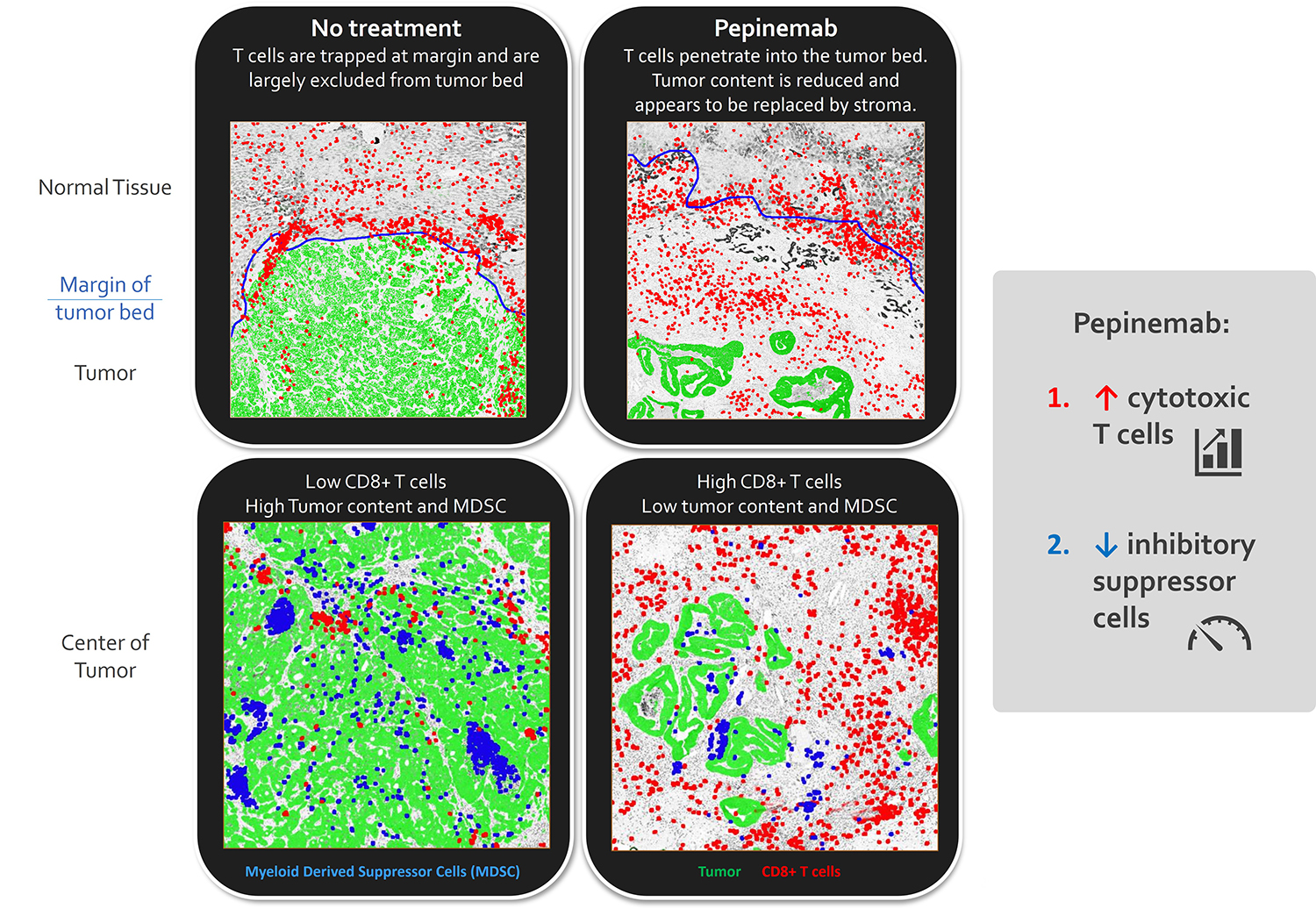
Immunohistochemical staining of colorectal tumors from patient treated with pepinemab shows an increase in CD8+ T cells that infiltrate the interior of the tumor and a reduction in myeloid suppressor cells.
SEMA4D is broadly expressed in many human cancers, and its expression correlates with invasive human disease. SEMA4D regulates the movement and differentiation of immune and vascular cells in the tumor environment. Antibody blockade of SEMA4D increases immune infiltration and activity in tumors and promotes durable tumor rejection in preclinical melanoma, colon, breast, and head & neck cancer models.
Preclinical studies demonstrated high concentrations of SEMA4D expressed at the invasive margin of tumors which serves to immobilize immune cells and restrict infiltration into tumor. Antibodies against SEMA4D neutralize this barrier and “open the gates” of the tumor to the immune system. This facilitates anti-tumor immune responses and results in reduced tumor burden in animals.
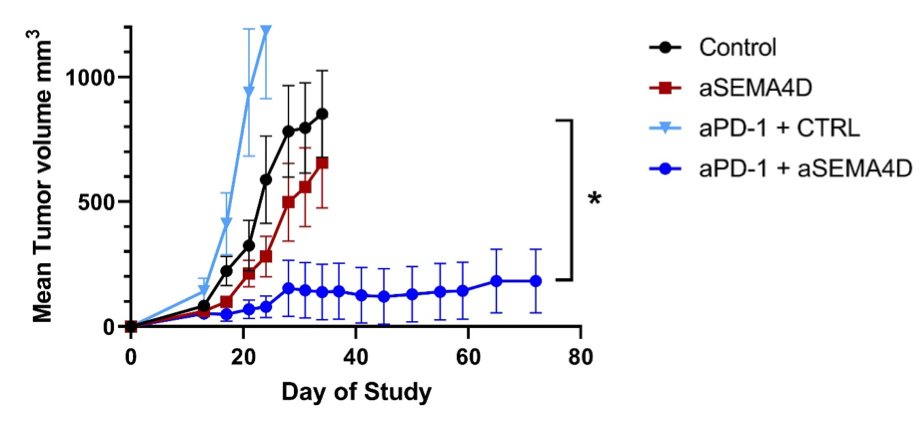
Administration of anti-SEMA4D antibody changes the tumor microenvironment by increasing infiltration of many types of immune cells that orchestrate a complex response. Preclinical data suggest that antibody blockade of SEMA4D not only greatly increases the number of immune killer cells that can attack the tumor, it also reduces the production of immunosuppressive cells that could otherwise interfere with this activity. Because, in order to survive and grow, tumors need to overcome the first line of defense against cancer, the protective immune system, they employ multiple immune evasion mechanisms. In addition to SEMA4D associated mechanisms, it is now well known that tumors express checkpoints like PD-L1 that can prevent activation of immune cells by binding to their matching PD-1 receptors. Checkpoint inhibitors have been developed to block the PD-1/PD-L1 signaling pathway. It is expected that an effective cancer treatment will likely need to overcome multiple tumor defenses. We, therefore, investigated the use of SEMA4D blocking antibody in combination with a checkpoint inhibitor. We detected strong synergy between the two anti-cancer agents. Optimal activity was observed when animals were treated with the combination of anti-SEMA4D antibody together with a checkpoint inhibitor.
Click to enlarge
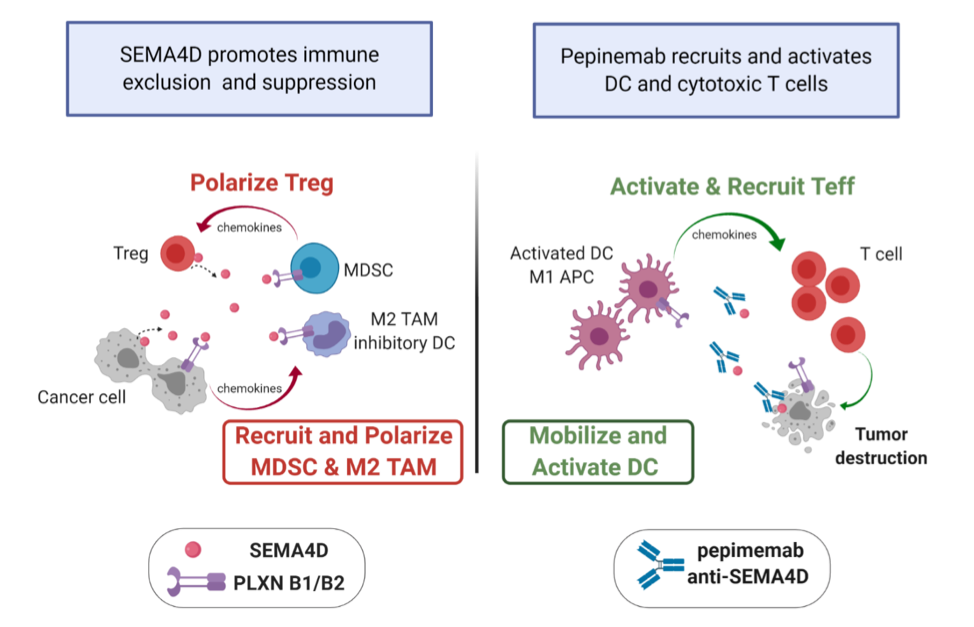
Effects of anti-SEMA4D therapy on the tumor microenvironment. SEMA4D blockade reprograms suppressive myeloid cells into activated antigen presenting cells that recruit and activate cytotoxic CD8+ T cells. This unique mechanism of action complements rational combination therapies to drive productive anti-tumorigenic effects. ((APC – Antigen Presenting Cell, DC- dendritic cell, MDSC – myeloid suppressor cell, Teff- Effector T cell, Treg – Regulatory T cell, CD8 – CD8+ T cell, TAM – Tumor associated macrophage). Graphics created with Biorender.com
Vaccinex publication describing preclinical studies of anti-SEMA4D:
Evans, et al. Cancer Immunol Res. 2015 Jun;3(6):689-701 (link)
Clavijo, et al. Cancer Immunol Res. 2019 Feb;7(2):282-291 (link)
Figure 1
Spatial and phenotypic reorganization of antigen presenting cells in mouse colon tumors:
Click to enlarge
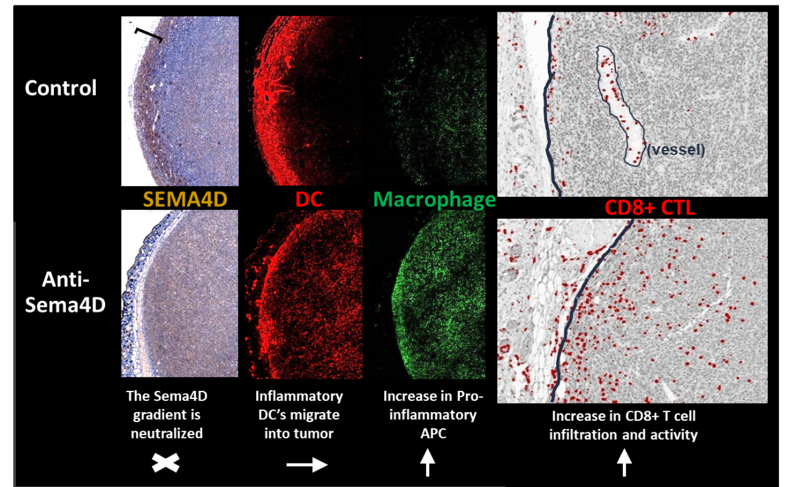
Colon26 tumors were collected on day 29 from Balb/c mice treated with Control IgG1 or anti-SEMA4D (MAb67). Serial sections were stained for expression of SEMA4D (left panel), CD11c (red) and F4/80 (green) (middle panels), merge is shown in right panel. A region of strongly SEMA4D-positive tumor and stroma was observed in Control tumors, indicated by brackets. Both SEMA4D gradient and distribution of monocytes and T cells were altered by SEMA4D blockade. Visual assessment suggests an increase in infiltration of F480+CD11c+ DCs. See Evans, et al. Cancer Immunology Research (link) for additional details.
Figure 2
Click to enlarge
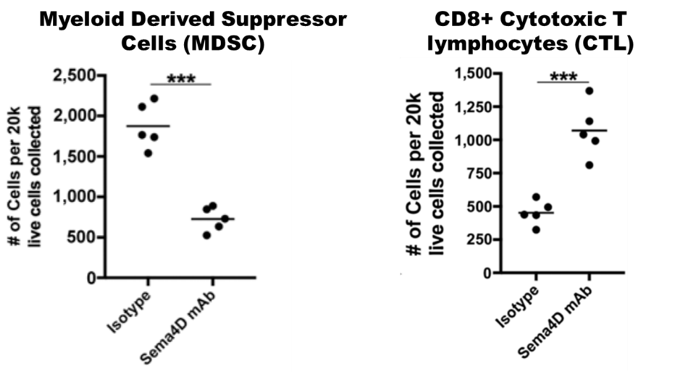
Mice carrying MOC1 head and neck tumors were treated with anti-SEMA4D or control antibodies for 1 month. SEMA4D antibody reduces recruitment and activity of myeloid suppressor cells and increases infiltration and activity of CD8+ T cells. See Clavijo, et al. Cancer Immunology Research (link) for additional details.
Figure 3
Click to enlarge
Results suggest synergy with immune checkpoint antibodies
Anti-PD-1 promotes expanded responses to tumor antigens while pepinemab (VX15/2503) acts synergistically to “open the gates” of the tumor to immune infiltration and also to reduce immunosuppressive cells. In preclinical studies, anti-SEMA4D enhances activity of numerous immunotherapies, including anti-PD-1 (above), anti-PD-L1, anti-CTLA-4, anti-LAG3, anti-TGFβ, etc. More data is available in Evans, et al. Cancer Immunology Research (link).
Vaccinex has successfully completed a two-center Phase I, multiple ascending dose clinical trial of pepinemab in 42 adult patients with advanced solid tumors. Pepinemab was found to be well tolerated at the highest dose level tested, 20 mg/kg.
Studies of pepinemab in cancer are being conducted by both Vaccinex and academic collaborators. The program has generated excitement in the scientific community and was highlighted at the ASCO-SITC Clinical Immuno-Oncology Symposium in February 2020 (link). In total, six oncology studies will evaluate the safety and efficacy of pepinemab.
Study 1.
An ongoing clinical study in collaboration with Merck to test pepinemab in combination with KEYTRUDA® to treat patients with recurrent or metastatic Head & Neck Squamous Cell Carcinoma (HNSCC).
KEYNOTE-B84 press releases
Link to ClinicalTrials.gov Identifier: NCT04815720
Study 2.
Vaccinex has completed a a Phase Ib/II study of pepinemab in combination with avelumab, a human anti-PD-L1 IgG1 monoclonal antibody, in patients with advanced (stage IIIB/IV) non-small cell lung cancer (NSCLC) in a clinical collaboration with Merck KGaA, Darmstadt, Germany. The clinical trial, short-named “CLASSICAL – Lung”, is a multi-center, open-label study designed to evaluate the safety and potential efficacy of the combination of pepinemab (VX15/2503) and avelumab in patients with advanced NSCLC.
Results of this study have been published in 2021. In both patients that have not previously received immunotherapy and those whose tumors progressed following treatment with single-agent checkpoint inhibitor, increased CD8+ cytotoxic T cell activity and either reduction or stabilization of tumor burden was observed in a majority of patients. The objective responses for patients that received the combination treatment appeared to be 2 to 2.5 times higher than those for a comparable population of patients who received single agent Bavencio® (avelumab) in a prior study. Progression-free survival also appeared to be extended by combination therapy.
CLASSICAL-Lung press releases
Link to ClinicalTrials.gov Identifier: NCT03268057
Link to publication in Clinical Cancer Research
Figure 4
Click to enlarge
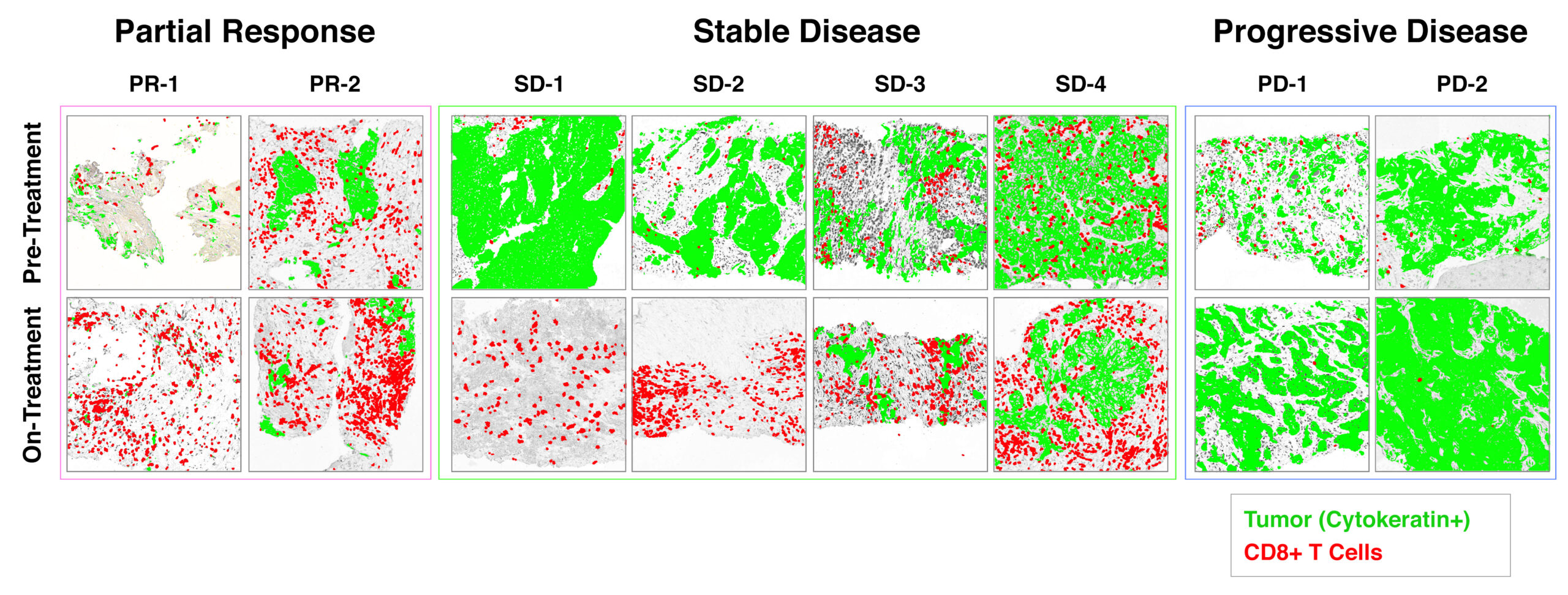
Rapid increase in infiltration of CD8+ CTL corresponds with durable clinical benefit.
Biopsies were collected from patients enrolled in the CLASSICAL-Lung trial after ~ 1 month of treatment and compared to baseline pre-treatment biopsies of the same lesion. These data were reported at ASCO Annual Meeting 2020 (link).
Study 3 and 4.
Three Investigator Sponsored Studies (IST) are being managed by clinicians at the Winship Cancer Institute of Emory University to evaluate the effect of diverse treatment regimens including pepinemab on the immune profile in the tumor microenvironment.
- Study 3: A Phase 1 clinical trial evaluating pepinemab as a single agent and in combination with ipilimumab or nivolumab in pre-surgical patients with resectable head and neck cancer. Link to ClinicalTrials.gov Identifier: NCT03690986
- Study 4: A Phase 1 clinical trial evaluating pepinemab as a single agent and in combination with ipilimumab or nivolumab in pre-surgical patients with resectable melanoma. Link to ClinicalTrials.gov Identifier: NCT03769155
Study 5.
A study managed by the Children’s Oncology Group and National Cancer Institute, entitled A Phase 1/2 Study of VX15 (IND#136181) in Children, Adolescents, or Young Adults with Recurrent or Relapsed Solid Tumors, enrolled children with advanced solid tumors and adolescents/young adults with osteosarcoma for treatment with single agent pepinemab. The trial was initiated in January 2018 and was completed in April 2020.
Link to ClinicalTrials.gov Identifier: NCT03320330