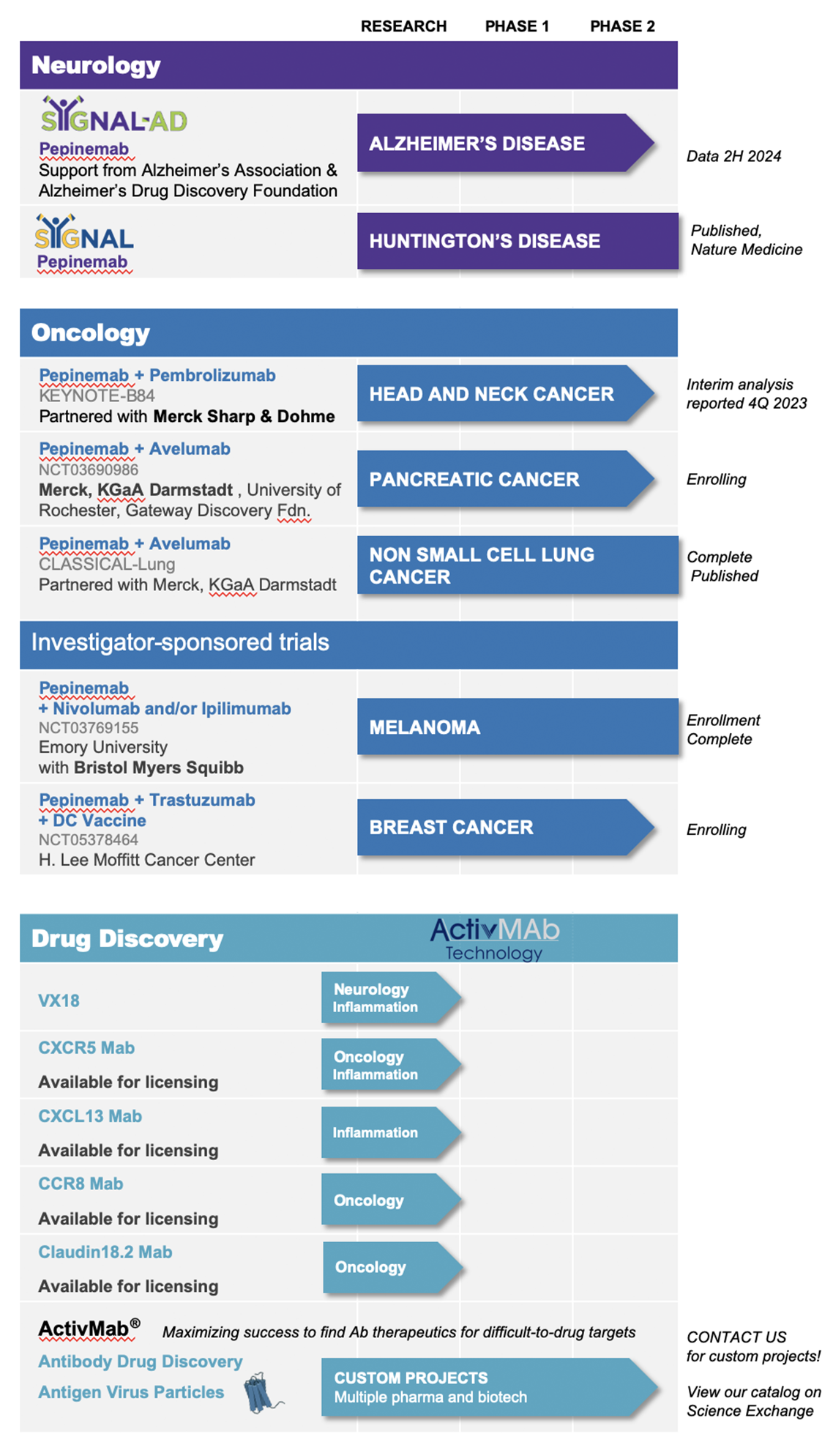
Vaccinex’s lead clinical programs are focused on inhibition of semaphorin 4D (SEMA4D), a novel target and an important biological effector whose expression is implicated in the progression of a number of neurological diseases and cancers.
Pepinemab, a monoclonal antibody targeting SEMA4D is Vaccinex’s most advanced clinical candidate and is currently being studied in clinical trials for head and neck cancer (R/M HNSCC), Huntington’s Disease (HD), Alzheimer’s Disease (AD) and in addition to recently completed phase 2 clinical trials in Huntington’s Disease (HD) and non-small cell lung cancer (NSCLC). Vaccinex is actively planning for a phase 3 trial in HD. Pepinemab also has future potential as a treatment for other cancers, as well as neurodegenerative disorders, including multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Rett Syndrome.