Semaphorin 4D (SEMA4D) plays a role in multiple cellular processes that contribute to the pathophysiology of neuroinflammatory/neurodegenerative diseases. SEMA4D is, therefore, a uniquely promising target for therapeutic development.
Pepinemab is a novel monoclonal antibody that blocks the activity of SEMA4D, and preclinical testing has demonstrated the beneficial effects of anti-SEMA4D treatment in a variety of neurodegenerative disease models. Vaccinex is committed to the development of this important antibody that has the potential to help people with different neurodegenerative disorders that share common mechanisms of pathology.
Note: Pepinemab (VX15/2503) is an investigational drug currently in clinical studies. It has not been demonstrated to be safe and effective for any disease indication. There is no guarantee that pepinemab (VX15/2503) will be approved for the treatment of any disease by the U.S. Food and Drug Administration or by any other health authority worldwide.
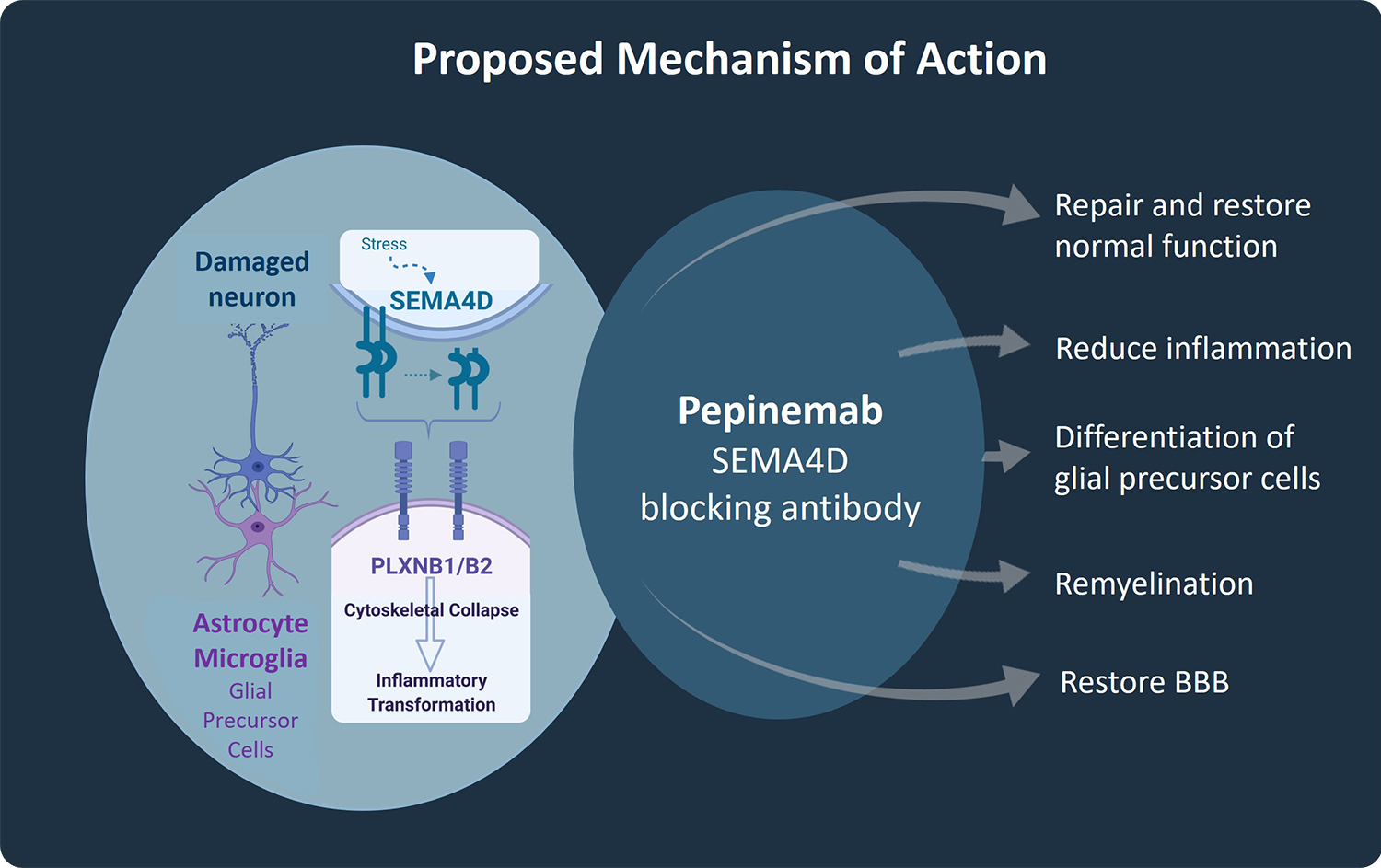
The mechanism of action of pepinemab involves inhibition of SEMA4D, which controls the activity of several different cell types important to normal brain function. These include astrocytes and microglia, the main innate inflammatory cells of the brain whose chronic activation is believed to contribute to neurodegenerative processes, and oligodendrocyte precursor cells (OPCs), that have the potential to remyelinate damaged nerves, but which are immobilized and prevented from differentiating by SEMA4D. In addition, accumulation of SEMA4D at the blood-brain barrier leads to breakdown of tight junctions between endothelial cells that maintain its integrity and function to preserve required concentrations of large and small molecules in the brain.
Astrocytes and microglia are key components of the brain that support neurons and shape neural networks by regulating formation and maintenance of synapses. Astrocytes cradle synapses through process extensions that express glutamate receptors and are involved in recycling 80% of free glutamate, the main transmitter of excitatory nerve impulses. Astrocytes are also an important part of the neurovascular unit that mediate glucose uptake from circulation. This allows astrocytes to play a key role in coupling the need for glucose transport with excitatory synaptic activity.
Importantly, under conditions of physiological stress or injury, astrocytes and microglia transition from their normal function to an inflammatory state that can trigger or exacerbate neurodegeneration. In this inflammatory state, astrocytes abandon their normal functions, including through down-regulation of both glutamate receptors and glucose transporters, and, instead, initiate secretion of inflammatory cytokines. Expression of the Huntington disease mutation in astrocytes alone has been shown to be sufficient to trigger the disease phenotype in an animal model.
In preclinical research, we demonstrated that SEMA4D is upregulated in neurons in response to stress or injury such as occurs during underlying progression of Huntington’s disease, Alzheimer’s disease, and Rett Syndrome. Astrocytes and microglia express high affinity receptors for SEMA4D and are in close proximity to neurons. SEMA4D expression by neurons can, therefore, trigger inflammatory transformation of astrocytes. This is the therapeutic rationale for use of pepinemab to treat neurodegenerative disorders. Pepinemab binds to SEMA4D and inhibits its activity, thereby preventing the increase in damaging inflammatory transformation and preserving essential normal astrocyte functions, including glucose transport and recycling of neurotransmitters.
Figure 1
Click to enlarge
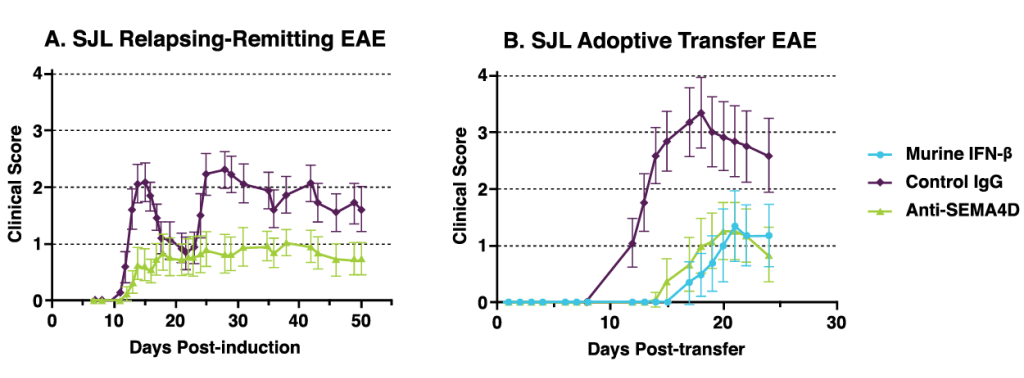
Anti-SEMA4D antibody treatment significantly improves clinical scores in two different rodent models of experimental autoimmune encephalomyelitis (EAE) an animal model of MS. Data published in Smith et al, 2014, Neurobiology of Disease (73); 254-268.
Figure 2
Click to enlarge
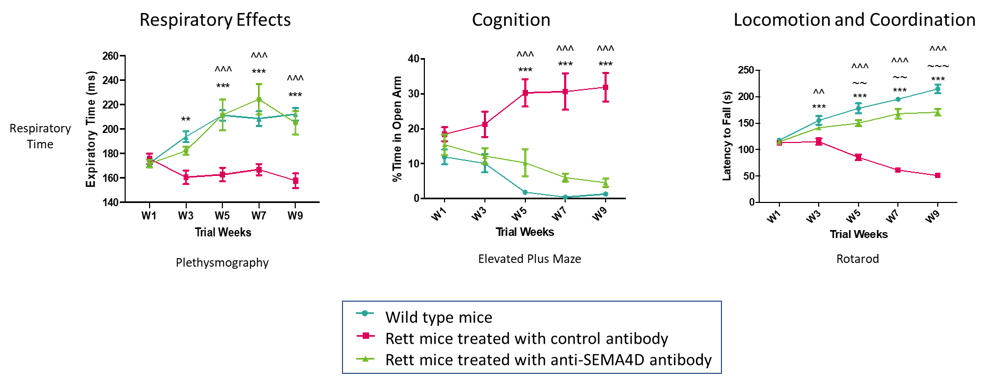
Anti-SEMA4D treatment significantly inhibits behavioral deficits in Mecp2T158A/y mutant mice, a transgenic model of Rett Syndrome. The blue lines show behavior in normal control mice while red lines represent the transgenic Rett Syndrome animals. Green lines represent the animals that received preventive therapy with anti-SEMA4D antibody. Data published in Mao et al, 2021, Int. J Mol. Sci (22): 9465.
Figure 3
Click to enlarge
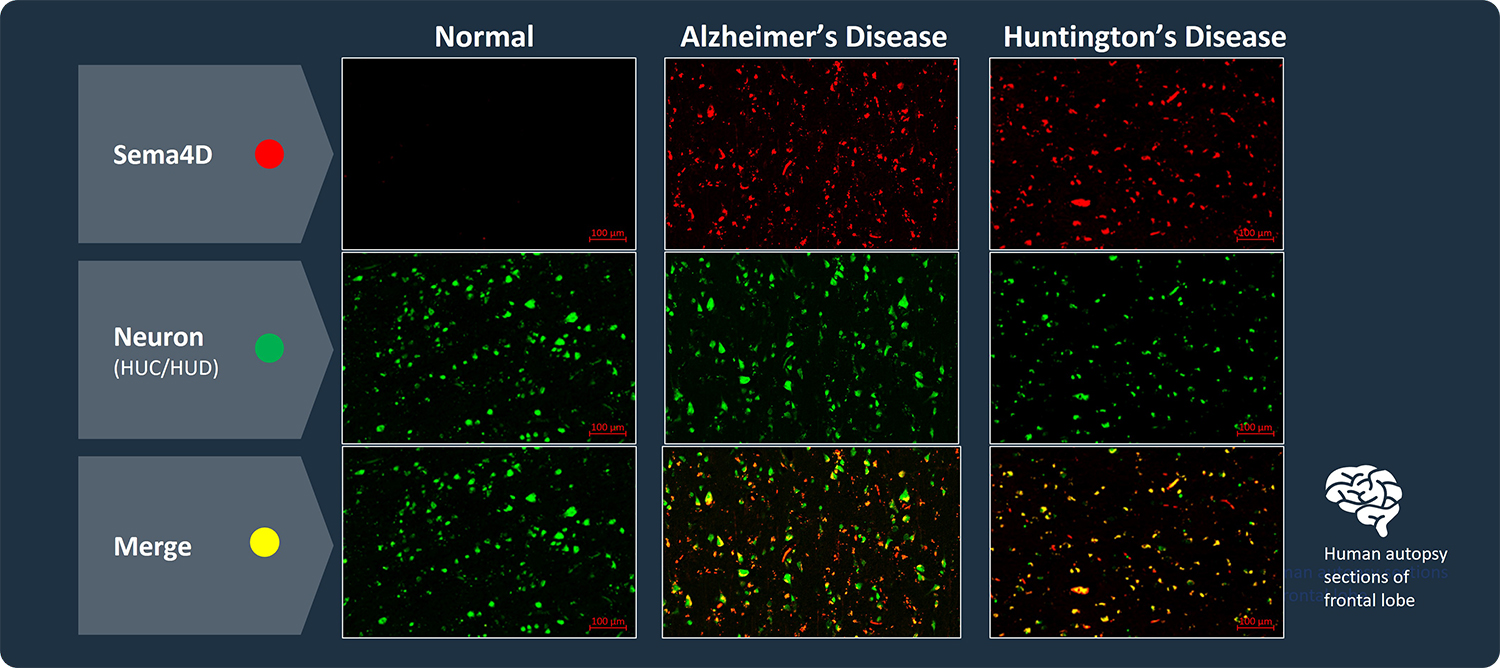
SEMA4D is progressively upregulated in neurons of subjects with Huntington’s Disease (HD) and Alzheimer’s Disease. Autopsy sections of frontal lobe are shown, stained for expression of SEMA4D (red), neuronal marker HUC/HUD (green), and co-expression of SEMA4D in neurons is evident in merge, which appears as yellow to orange with increasing SEMA4D expression (Evans et al. J Neuroinflammation 2022). Additional preclinical data demonstrating activity of SEMA4D antibody in a mouse model of Huntington’s disease has been published in Southwell et al. Neurobiol Dis. 2015 Feb 3; 76:46–56.
Vaccinex is developing pepinemab as a potential treatment for Huntington’s disease (HD), a fatal neurodegenerative disease in which symptoms typically appear around age 30-50. Approximately 30,000 people in the United States have manifest symptoms of HD, but 100,000–200,000 are believed to have inherited the genetic mutation and would be expected to eventually develop symptoms. There is no FDA-approved disease-modifying treatment available for HD. By targeting SEMA4D, considered to be a key driver of neuroinflammation, pepinemab has the potential to be the first disease-modifying therapy available to patients contending with HD.
The FDA’s Division of Neurology Products has granted both Orphan Drug designation and Fast Track designation to pepinemab (VX15/2503) for Huntington’s disease.
Vaccinex has recently completed the SIGNAL trial, a late-stage clinical study of pepinemab for HD (NCT02481674) published in Nature Medicine, 2022. The trial was a multi-center, randomized, double-blind and placebo-controlled study examining the effect of pepinemab in both early manifest and late prodromal subjects. Endpoints of the study included safety and tolerability, clinical and behavioral outcomes, as well as measures of brain volume, metabolic activity and inflammation. Pepinemab was well-tolerated with low treatment discontinuation or study drop-out rates over the extended 18-month treatment period. As for any well-designed phase 2 study, the main goal of SIGNAL was to identify a patient population that can benefit from the selected treatment and to characterize endpoints that can be employed to evaluate treatment benefit in this population. Although the study did not meet pre-specified co-primary endpoints, post-hoc stratification of analysis in patient populations showed markedly greater ability to detect beneficial clinical changes in patients who were somewhat more advanced in disease progression at the time treatment was initiated. We believe that this reflects the difficulty of discerning clinical changes very early in disease progression because of the slow rate of change early in disease which then accelerates over time. In addition, the results of each of the two cognitive assessments demonstrated a strong trend for beneficial change (One Touch Stockings, p=0.028 and Paced Finger Tapping task, p=0.06). These cognitive assessments reflect changes in planning ability and memory associated with disease progression. Further exploratory analysis also indicate that pepinemab has the potential to provide cognitive benefit and slow brain atrophy in HD patients. Key observations from pre-specified and post-hoc analyses include:
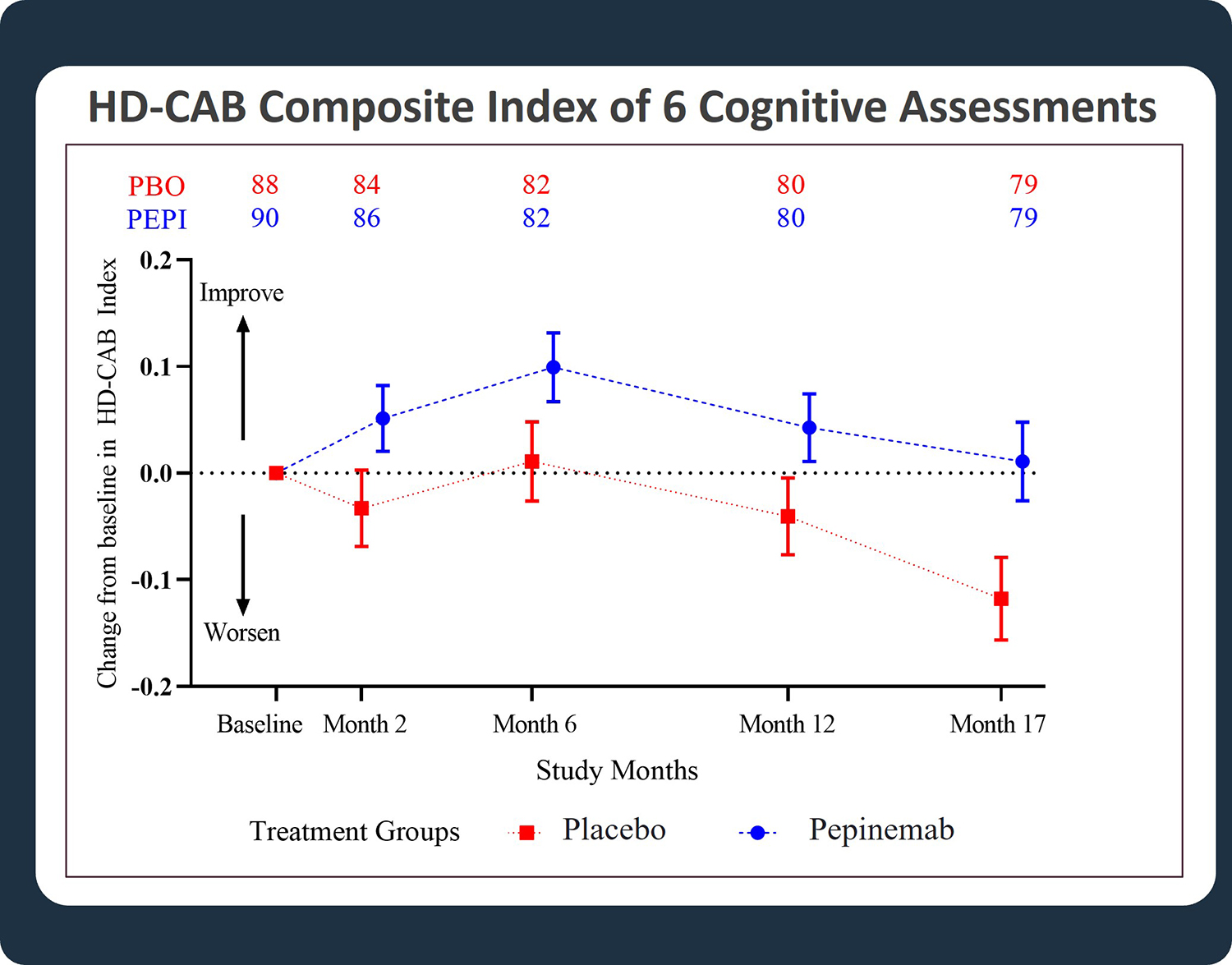
- Highly significant treatment benefit for pepinemab as reflected in the pre-specified exploratory analysis of HD-Cognitive Assessment Battery (HD-CAB) Composite Index (Figure 1)
- A further signal of benefit was provided by post-hoc analysis of treating physicians’ Clinical Global Impression of Change (CGIC) in a subpopulation of patients with somewhat more advanced disease progression (with baseline total functional capacity of 11)
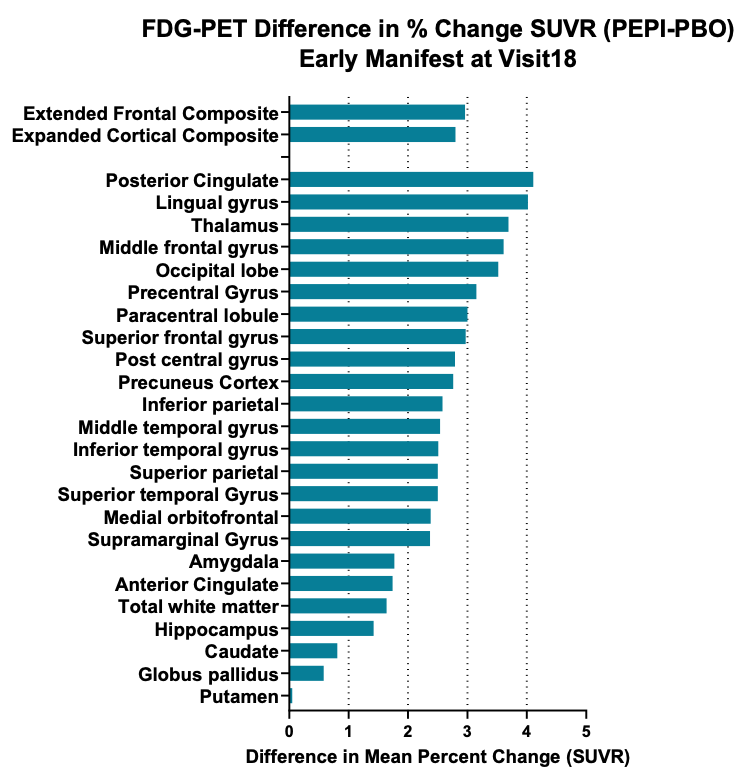
- Pre-specified exploratory volumetric MRI analysis of brain in patients with early manifest disease demonstrate treatment related reduction in brain atrophy and FDG-PET imaging shows increased brain metabolic activity (Figure 2)
Figure 1
Click to enlarge
Significant improvement in cognitive scores of HD Cognitive Assessment Battery (HD-CAB) composite index was observed in HD subjects with early manifest disease. The difference between placebo (PBO) and pepinemab (PEPI) treatment was highly significant, with a one-sided p-value = 0.007. (Feigin et al, 2022)
Figure 2
Click to enlarge
Graphical representation of effect size (changes in FDG-PET signal (SUVR, standardized uptake value ratio as a percentage of baseline over an 18-month treatment period) in a total of 24 brain regions of interest and two composite areas, the majority of which resulted in significant difference following treatment with pepinemab, with a two-sided *p-value <0.05. (Feigin et al, 2022)
In addition to its potential in HD, pepinemab holds promise as a treatment for Alzheimer’s disease (AD). Decline in FDG-PET signal, which pepinemab has been shown to prevent in patients with HD, has been established as an early prognostic indicator of cognitive decline in AD. SEMA4D has also been found to be upregulated in cortical neurons of patients suffering from AD which we believe can trigger a chronic inflammatory cycle.
As in Huntington’s disease, SEMA4D is upregulated on neurons and can signal to astrocytes, impairing normal support functions such as glucose transport. There is clear evidence demonstrating that impaired glucose metabolism is associated with AD brain pathology and symptoms, providing a strong rationale for anti-SEMA4D treatment with pepinemab, which could potentially lead to a reduction or slowing of brain pathology and cognitive symptoms in AD.
Vaccinex has initiated the SIGNAL-AD trial, a Phase 1, placebo-controlled study of pepinemab for the treatment of AD (NCT04381468).
Vaccinex’s AD program is supported by awards from the Alzheimer’s Association ($750,000) and the Alzheimer’s Drug Discovery Foundation (up to $3 million).
Because disease progression in MS is associated with neuroinflammation, there is strong evidence suggesting pepinemab could serve as a potential treatment option for these disorders.
Vaccinex has successfully completed a Phase I, multi-center, randomized, double-blind, placebo-controlled, single-ascending dose clinical trial of pepinemab (VX15/2503) anti-SEMA4D antibody in 50 adult patients with multiple sclerosis. While 10 patients were treated with placebo, 40 patients were treated with single doses of pepinemab (VX15/2503) ranging from 1 to 20 mg/kg. Pepinemab (VX15/2503) was well tolerated and no Maximum Tolerated Dose (MTD) was reached. (Link to publication)