Immunotherapy in Patients with Resectable Solid Tumors – Integrated Biomarker Studies
Trial Purpose
These randomized phase I trials are intended to study pepinemab (VX15/2503) in combination with or without ipilimumab or nivolumab. The purpose is to determine how different treatments affect immune biomarkers in the tumor micro-environment and peripheral blood in patients with various solid tumor cancers.
Vaccinex’s approach is to reprogram immune suppression in the tumor microenvironment by potentially reducing the function and number of suppressive cells, such as myeloid derived suppressor cells, and thereby may overcome immune resistance mechanisms and increase activity of cytotoxic T cells.
Immunotherapy in Stage I-IVA resectable head and neck squamous cell cancer
Trial Details
About the Trial
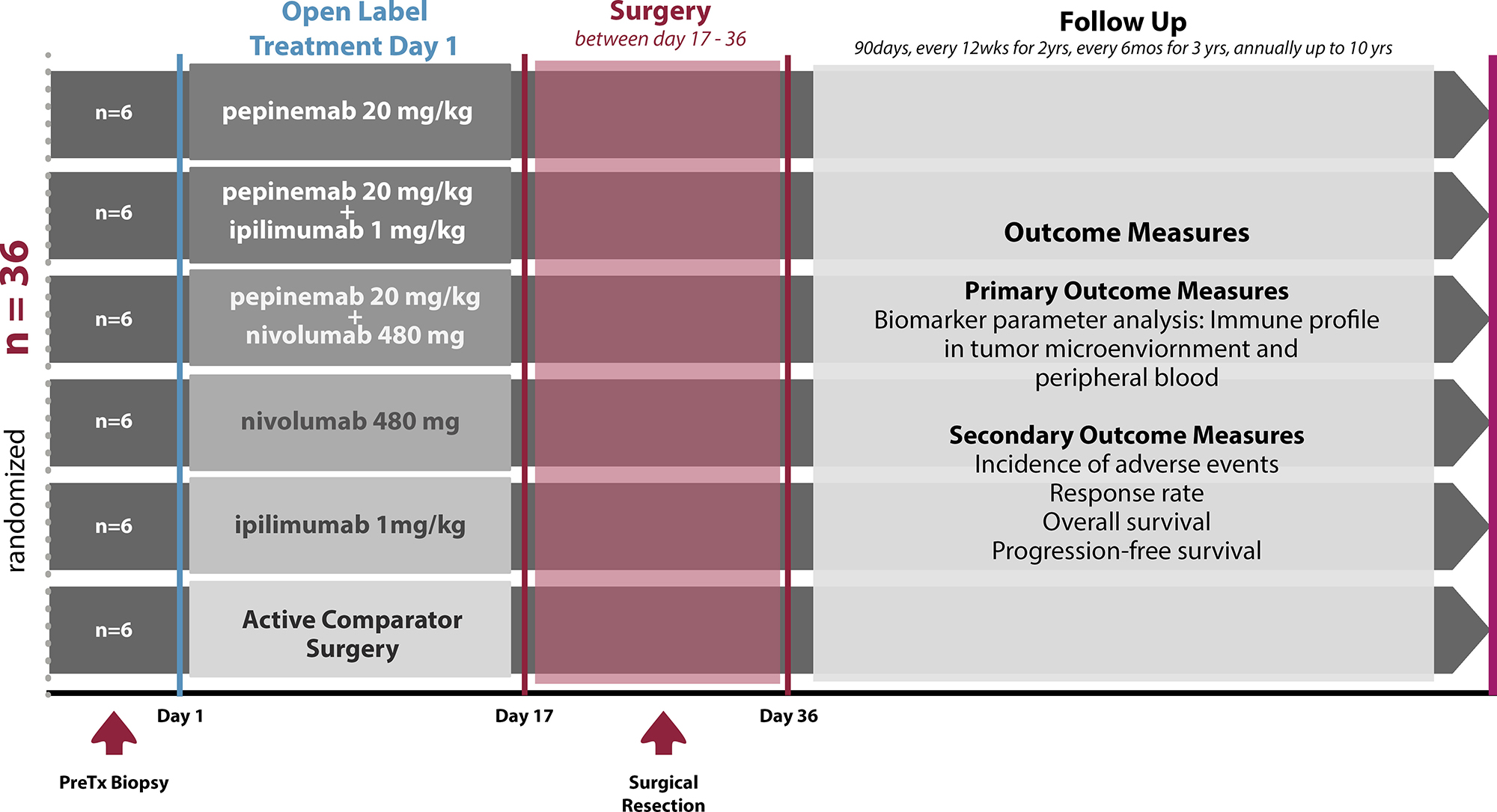
All patients who enroll in the trial will receive standard of care surgery. Patients will be randomized to 1 of 6 groups to receive standard of care surgery, or immunotherapy with pepinemab, ipilimumab, nivolumab, pepinemab plus ipilimumab, or pepinemab plus nivolumab, prior to surgery.
Phase
Phase 1
Dates
Start Date
November 1, 2018
Estimated Study Completion Date
July 14, 2022
Sponsor
Emory University
Collaborators
Vaccinex Inc.
Products
pepinemab
Nivolumab
Ipilimumab
Trial Design
Click to enlarge
Immunotherapy in Resectable Stage IIIB-D Melanoma
Trial Details
About the Trial
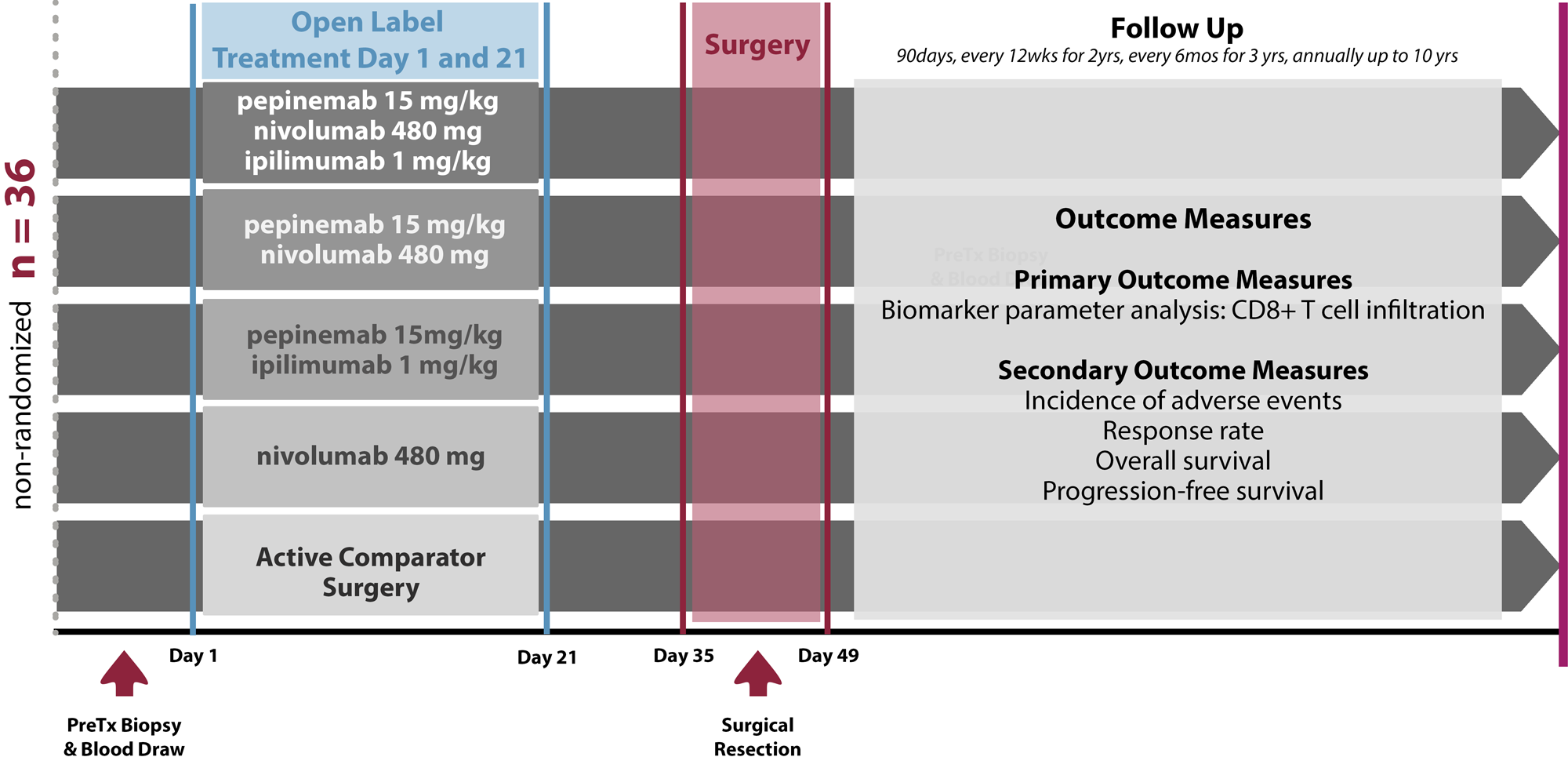
All patients who enroll in the trial will receive standard of care surgery. Patients will be randomized to 1 of 5 arms to receive standard of care surgery, or two additional immunotherapy treatments on days 1 and 21 plus surgery between days 35-49. Immunotherapy treatment arms include pepinemab + nivolumab, pepinemab + ipilimumab, pepinemab + nivolumab plus ipilimumab, or nivolumab alone. Biomarker analysis and pathologic response will be evaluated.
Phase
Phase 1
Dates
Start Date
December 13, 2018
Estimated Study Completion Date
December 31, 2021
Sponsor
Emory University
Collaborators
Vaccinex Inc.
Products
pepinemab
Nivolumab
Ipilimumab
Trial Design
Click to enlarge
Only a qualified healthcare professional can determine your eligibility.
However, this information may be useful in starting a conversation with your doctor.