Pepinemab in Combination with Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE B84)
Clinical Trial Evaluating the Safety and Efficacy of Pepinemab in Combination with Pembrolizumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck
Vaccinex seeks to build clinical programs that beneficially impact patient care. With that goal in mind, we are developing a pipeline of novel candidate therapeutics that we hope will improve upon existing therapies and offer patient treatment options that improve their quality of life.
Trial Purpose
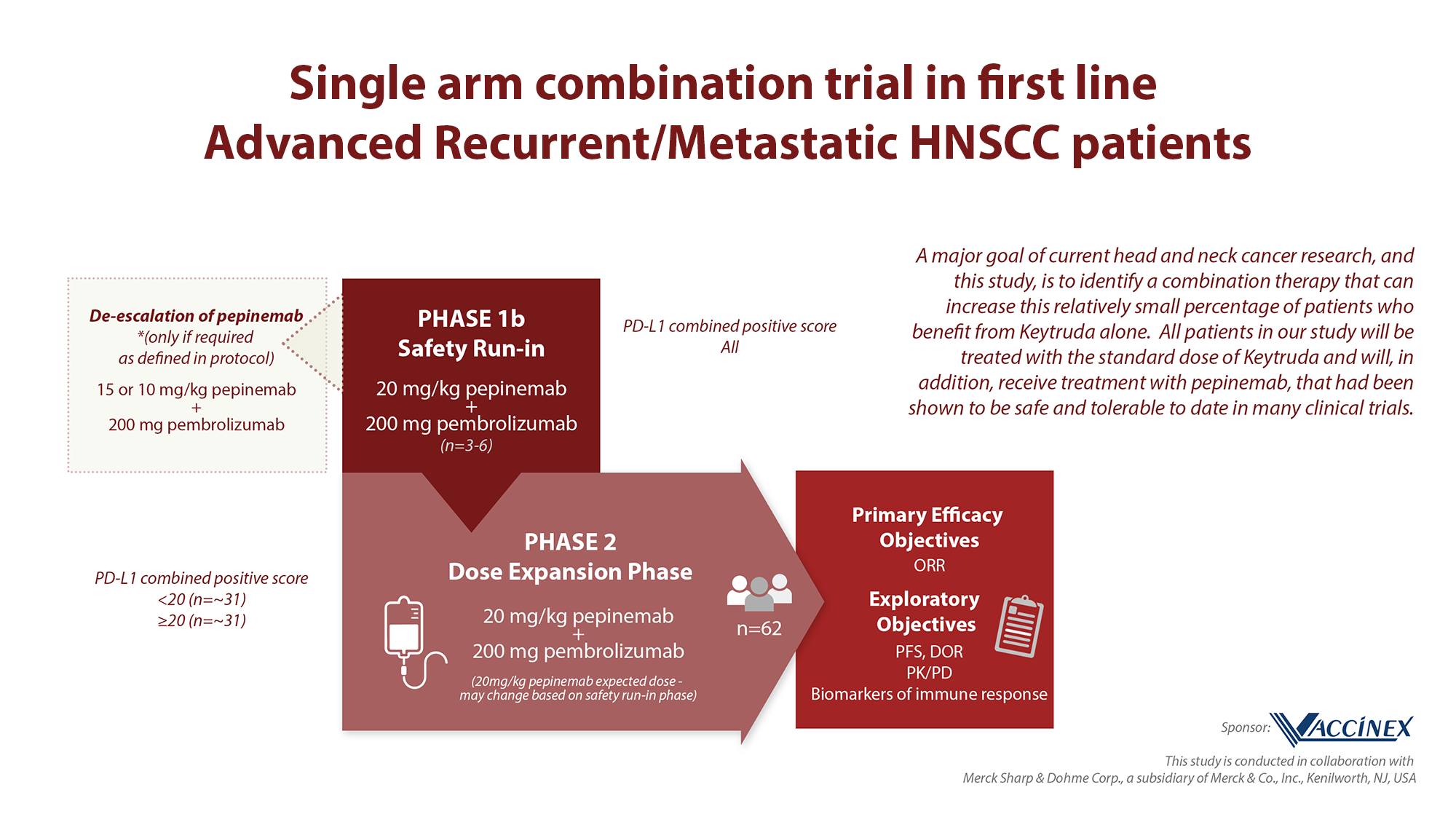
The purpose of the study is to evaluate the safety and tolerability of pepinemab in combination with pembrolizumab and determine a recommended Phase 2 dose (RP2D) in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). The primary hypothesis being tested is whether the combination of pepinemab + pembrolizumab will be safe and provide clinical benefit in first-line treatment of patients with R/M HNSCC.
Pembrolizumab is an FDA-approved anti–PD-1 immunotherapy to treat certain patients with head and neck squamous cell cancer, and may be used alone or in combination with platinum-based chemotherapy.
Vaccinex’s approach is to reprogram immune suppression in the tumor microenvironment by potentially reducing the function and number of suppressive cells, such as myeloid derived suppressor cells, and thereby may overcome immune resistance mechanisms and increase activity of cytotoxic T cells.
Trial Details
About the Trial
This Phase 1/2 trial will evaluate the safety and tolerability of pepinemab in combination with pembrolizumab and determine a recommended Phase 2 dose (RP2D) in up to 65 patients with advanced, recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). The Phase 1 safety run in segment is now complete. In the Phase 2 expansion segment, patients will be treated with pepinemab IV starting at 20 mg/kg and pembrolizumab at 200 mg IV, every 3 weeks. Subjects will undergo evaluation for extent of disease at baseline, and ~ every 6 weeks through year 1, and every 9 weeks thereafter. All patients enrolled in the study will be treated with the standard dose of pembrolizumab and in addition, receive treatment with pepinemab.
Please consult ClinicalTrials.gov NCT04815720 for full details. Main inclusion criteria include:
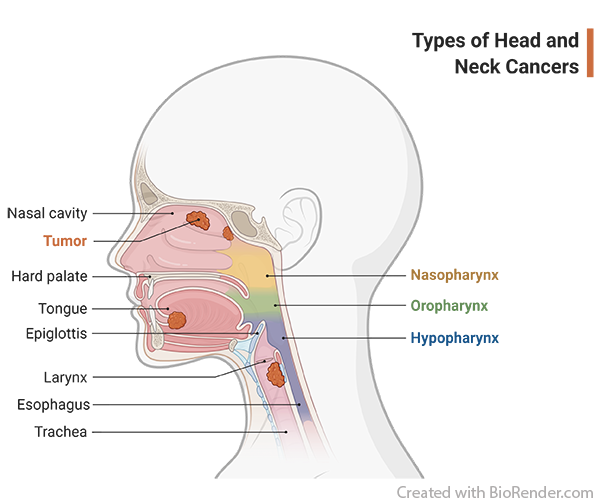
- Subjects must have histologically or cytologically confirmed HNSCC; eligible histologies include squamous cell carcinoma (SCC) of the oropharynx, oral cavity, hypopharynx and larynx.
- Have measurable disease per RECIST 1.1 as assessed by (the central imaging vendor OR the local site investigator/radiology).
- ECOG 0 or 1
- This trial is for first-line treatment for their recurrent or metastatic HNSCC, subjects who have received adjuvant systemic therapy or systemic therapy for locally advanced disease which was completed more than 6 months prior to study enrollment are eligible.
Phase
Phase 1/2
Dates
Actual Study Start Date
August 9, 2021
Estimated Primary Completion Date
September 4, 2023
Estimated Study Completion Date
September 4, 2023
Sponsor
Vaccinex Inc.
Collaborators
Merck Sharp & Dohme Corp.
Products
pepinemab + pembrolizumab
Only a qualified healthcare professional can determine your eligibility.
However, this information may be useful in starting a conversation with your doctor.