A Phase 1b/2 Trial of Immunotherapy With Avelumab and Pepinemab as Second Line For Patients With Metastatic Pancreatic Adenocarcinoma
Trial Purpose
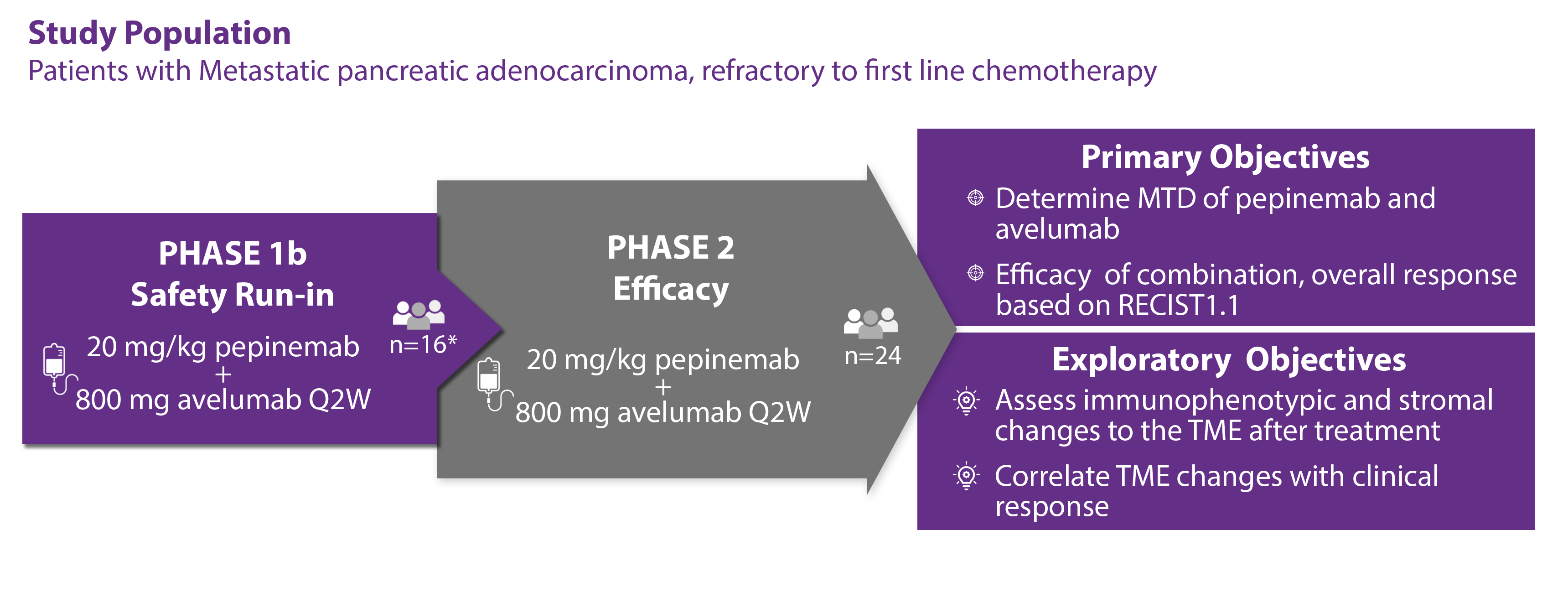
The purpose of the study is to evaluate the safety and clinical activity of pepinemab in combination with avelumab in patients with metastatic pancreatic adenocarcinoma that has progressed after first line chemotherapy.
Pancreatic adenocarcinoma is the second most common gastrointestinal malignancy in US with poor prognosis. Avelumab is a programmed cell death ligand-1 (PD-L1) blocking antibody for the treatment of various tumors.
Vaccinex’s approach is to reprogram immune suppression in the tumor microenvironment by potentially reducing the function and number of suppressive cells, such as myeloid derived suppressor cells, and thereby may overcome immune resistance mechanisms and increase activity of cytotoxic T cells.
The combination of pepinemab and avelumab has been shown to be well-tolerated in patients with lung cancer. Signs of anti-tumor response were seen in both patients that were IO naïve and patients that had failed prior IO therapy. In addition, on-treatment biopsies showed increased CD8+ T-cell infiltration in patients that had reduced or stabilized tumor burden. (https://pubmed.ncbi.nlm.nih.gov/33820783/)
Trial Details
About the Trial
This open label Phase 1/2 trial will evaluate the safety and tolerability of pepinemab in combination with avelumab and assess efficacy of combination therapy in up to 48 participants with metastatic pancreatic adenocarcinoma.
Phase 1b will determine the MTD defined via BOIN design targeting a 30% DLT event rate within a given dose combination, with cohort enrollments of 16-44 patients. Patients in Phase 1b will be treated with 800mg of Avelumab every 2 weeks and 20, 15 or 10mg/kg pepinemab every 2 weeks by intervienious injections. Accrual continues in Phase 1b untill the 16th pateint is evaluated for RECIST 1.1. Phase 2 enrollment begins once 16th evaluation passes Simon’s Tow State Rule. Dosing for Phase 2 will be defined by MTD from Phase 1b. Total number of patients between Phase 1b/2; n=16-68 with an estimated cumulative total of 48 patients.
Phase
Phase 1b/2
Dates
Study Start Date
December 10, 2022
Sponsor
Vaccinex, Inc.
Collaborators
University of Rochester
Merck KGaA